In This Article
- FDA Outlines Stricter Covid Vaccine Booster Approval Standards
- Details on COVID-19 Vaccination Policy
On Tuesday, the Food and Drug Administration announced its new regulatory guidance and plans for the COVID-19 vaccines. It is not going to recommend the COVID vaccine to most adults and healthy kids. It has set stricter policies, and only the high-risk group is going to get the vaccine booster shots.
An editorial article is published in the New England Journal of Medicine that reveals that the authorities of the FDA are going to change the vaccine booster recommendations, and this time, only those who require it the most are getting it.
Till now, there was the rule that every individual, six months or older, would get the COVID-19 vaccination that targets the latest circulating version of the virus. The COVID-19 virus is going through changes, and the old vaccinations are unable to control the growing risks; therefore, modifications have been made to the vaccination, too.
FDA Outlines Stricter Covid Vaccine Booster Approval Standards
There are changes in the FDA authorities, as we see that Dr. Vinay Prasad is the newly appointed head of the Center for Biologics Evaluation and Research. He and Dr. Martin Makary, the FDA commissioner, have explained how the FDA is going to make changes in its vaccine regulations. The young and healthy people are not getting the updated version of the COVID-19 vaccines.
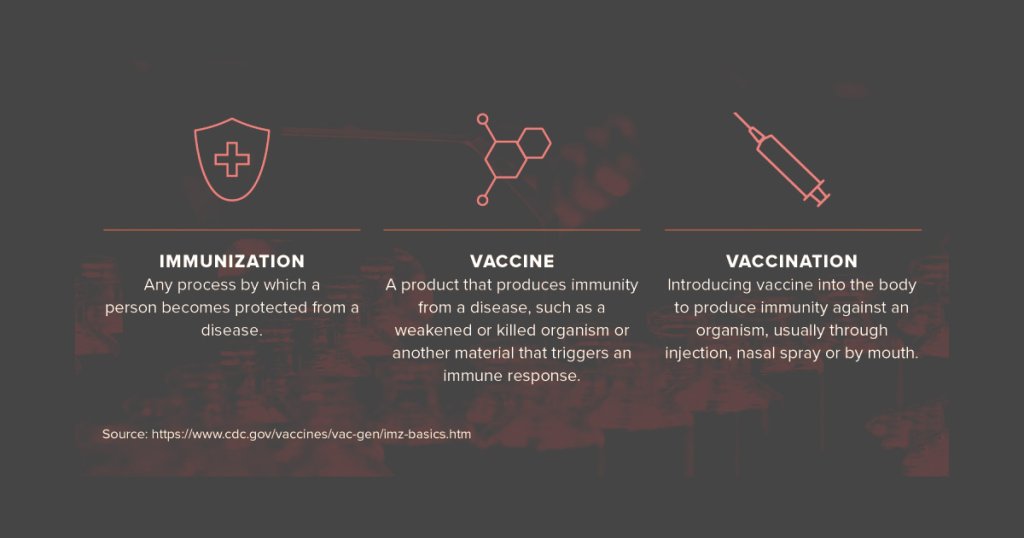
Viney writes that the new vaccine must pass through the placebo-controlled clinical trials; therefore, the real vaccine will be provided to certain people, and others will get an inactive substance, such as a saline shot. The FDA will then compare the results of both individual types, and this will be a big experiment.
In COVID and after that, the research and studies are made stronger using experimentation and the latest technologies like artificial intelligence. Every field requires the use of AI, particularly for web solutions where success is all about the position in the competition.
Weborik Hub witnesses the apps and websites that, after they have integrated the AI features, have made great progress, and the company has a great hand behind their success.
Details on COVID-19 Vaccination Policy
On Tuesday, in the later question-and-answer session about the new policy, Makary and Prasad talked about the plans. According to them, the healthy adults’ and children’s vaccination will no longer be approved in routine.
Right now, the vaccines are annually updated, and the FDA has decided not to carry this out in the future. Parsad says,
Instead of having a Covid strategy that’s year-to-year, why don’t we let the science tell us when we should change? The virus doesn’t have a calendar.
The current vaccine version is safe for adults and healthy people, so there is no need to spend the budget on the upgrade or to change the dose for healthy people. He says,
The FDA will approve vaccines for high-risk persons and, at the same time, demand robust, gold-standard data on persons at low risk
In the answer to a question, Makary mentioned,
These clinical trials will inform future directions for the FDA, but more important, they will provide information that is desperately craved by health care providers and the American people
It seems that all the medical fields are getting the low budget from the Trump administration; the COVID-19 vaccine is also the victim of upgrade development and the penalty of new vaccination availability because of the budget constraints.



